MHS Chemistry
Bonding Summary
Bonding
There are just under one-hundred elements that occur in nature, but there are
millions and millions of different compounds. Your hair, the air you breathe,
the ink in a pen, the components of paper, the metals and plastics making your
computer, and the water in the fountains are just a very few examples of the
chemicals that exist everywhere in everything you touch. For us to be able to
talk about and control them, we must be able to organize them. And we organize
them by what kinds of bonds hold them together. The bonds holding atoms together
give any chemical it's very basic general characteristics.
A chemical bond occurs when two atoms are held together by
mutual attraction to the same electrons. This attraction is balanced by the
repulsion of the nuclei for each other, and the repulsion of the electrons for
each other. Since each element is a unique combination of protons and electron
arrangements, atoms of each element have a slightly different attraction for
electrons. This relative attraction for shared electrons has been tabulated
as electronegativity, which is a 0 to 4 scale. The atom with
the strongest attraction for shared electrons is fluorine, with an electronegativity
of 3.98, and the lowest electronegativity is that of Francium (0.7). The
symbol for electronegativity is c
(that's "c" in the symbol font).
The type of bond formed between two atoms depends on their electronegativity.
Atoms with strong attractions for electrons are non-metals, and tend to form
anions (negative ions). Atoms with weak attractions for electrons
are metals, and tend to form cations (positive ions). Atoms
with moderate attraction for shared electrons are known as transition
metals. There is one family of atoms that have virtually no
attraction for electrons, but also do not give up the ones they have, called
the noble gases. [Click here
for a review of metals and non-metals and their properties.]
There are some atoms that do not have electronegativities listed. Most of these
the atoms are so rare that it has not been possible to gather experimental data.
A few of them (the noble gases, see below) do not form any compounds, and so
a relative attraction for shared electrons cannot be calculated.
Chemical bonds occur when electrons end up paired with each other, and the
bonded atoms always have lower total energy than the separated atoms.
Ionic Bonding
If two atoms have very different attractions for electrons, then one of them
will "steal" the electrons from the other. These two atoms are then
"stuck" together by their opposite charges, in what is known as an
ionic bond. Atoms in ionic compounds do not need exactly opposite
charges; for example, calcium chloride has the formula CaCl2
and consists of calcium ions with a 2+ charge and chloride ions with a 1- charge.
There will be enough of each ion so the overall charge is zero.
Also, ionic substances always have their ions in specific ratios (like
in calcium chloride above: 1 Ca : 2 Cl), but they do not exist as molecules.
Instead, they exist as a crystal lattice, which is a
regularly constructed arrangement of positive and negative ions. These
lattices can be any size, from sub-microscopic to many feet across, but
for a given compound, they all have the same chemical
properties.
Because an ionic compound does not exist as a molecules of a specific
size, we cannot calculate a molecular weight. We may still need
to know how much of a certain compound we have, so for ionic compounds
we calculate formula mass. This is calculated and
used the same way as molecular weight, but it tells us the mass of a single
"formula unit" of a substance. For table salt, even though
there are no specific Na-Cl pairs, we still add up the mass of one sodium
atom and one chlorine atom, because they make up the formula unit.
Ionic compounds that dissolve in water and break into their individual
ions are known as electrolytes because the resulting
solution conducts electricity. Ionic compounds that do not dissolve in
water are called non-electrolytes, and tend to involve
larger ions. Almost all ionic compounds are solids at room temperature.
[If you can think of one that isn't, e-mail
me.] |
 |
Covalent Bonding
Two atoms with similar strong attactions for electrons can't "steal"
them from each other, so they must "share" electrons. This is known
as a covalent bond. Generally, atoms will
form a covalent bond if they are both non-metals. Also, many metals (except
from the first two columns) will form covalent bonds with non-metals, because
even though they have opposite tendencies to form ions, they are not so different
that complete transfer of an electron will take place.
Although some covalent compounds dissolve in water, like sugar or vinegar,
their solutions do not conduct electricity because they do not break into ions.
It may seem that this describes a non-electrolyte ionic compound, but there
is another difference: covalent compounds usually form molecules,
which are the smallest unit of a compound with all the properties of that compound.
Unlike lattices, molecules have definite composition. A molecule of water has
the formula H2O, which means that there is a tiny unit
with exactly two hydrogen atoms connected to exactly one oxygen atom. A different
number of either atom would be a different compound with different properties.
Covalent compounds are often liquid or gas at room temperature, and the ones
that are solid are often soft or waxy.
Metallic Bonding
We have seen that a given pair of atoms can either both strongly attract electrons
(covalent bond), or one can strongly attract electrons away from the other (ionic
bond). There is a third possibility that occurs if neither atom involved has
a strong attraction for other electrons. These atoms are metals, and the resulting
situation is known as a metallic bond. In this case, many atoms
will be sharing valence electrons, but so weakly that the electrons do not "belong"
to any specific nucleus. The collection of atoms acts like a clump of chocolate
chip cookie dough, with each chip being a nucleus, and the dough being the electrons.
They are all held together, and they hold a consistent shape, unless you push
on them. In that case, the whole system deforms, but the same nuclei and electrons
are still there. That's a model for malleability.
We won't be worrying too much about metallic bonds, except to say that two
or more metals together form metallic bonds.
How Can We Tell?
There are two ways to decide which type of bond is involved in a given
compound. There is a "rule of thumb" method, and a "calculation"
method. They're both pretty easy. "Rule
of Thumb" means an easy pattern to remember.
| The "rule of thumb" method requires
you to mentally divide the periodic table into four regions, shown below. |
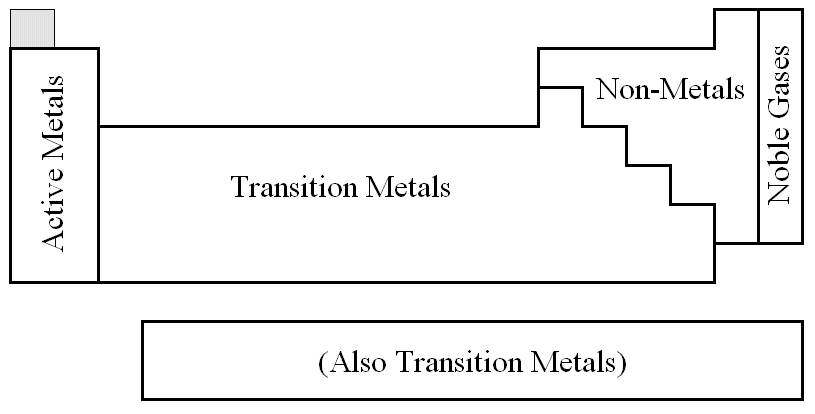 |
| active metal |
+ |
active metal |
makes |
a metallic |
bond |
| active metal |
+ |
transition metal |
makes |
a metallic |
bond |
| active metal |
+ |
non-metal |
makes |
an ionic |
bond |
| transition metal |
+ |
non-metal |
makes |
a covalent or ionic |
bond |
| transition metal |
+ |
transition metal |
makes |
a metallic |
bond |
| non-metal |
+ |
non-metal |
makes |
a covalent |
bond |
| anything |
+ |
noble gas |
makes |
no |
bond |
|
|
|
|
|
|
In other words, the closer together they are on the right
of the table, the more likely they are to form covalent bonds.
The calculation method requires a periodic table with
electronegativities listed (like the colorful ones you're all supposed
to have!). To find out what type of bond two atoms will form, subtract
their electrongativities (big minus small). If the difference is bigger
than 1.67, it's ionic. If the difference is less, it's covalent or metallic
(two metals). |
| |
| |
| Here's another way of thinking of it. |
| If two atoms have |
strong similar |
attractions for electrons, they will form |
covalent |
bonds |
(two non-metals) |
| |
weak similar |
attractions for electrons, they will form |
metallic |
bonds |
(two metals) |
| |
very different |
attractions for electrons, they will form |
ionic |
bonds |
(metal + non-metal) |
| If any atom has |
NO |
attractions for electrons, they will form |
NO |
bonds |
(noble gases) |
Try these:
| |
type of bond using "rule of thumb" method |
type of bond using calculation method |
| Li & Cl |
________________________ |
________________________ |
| C & Cl |
________________________ |
________________________ |
| Fe & S |
________________________ |
________________________ |
| Ba & I |
________________________ |
________________________ |
| N & O |
________________________ |
________________________ |
| Ga & Br |
________________________ |
________________________ |
| Fe & Ne |
________________________ |
________________________ |
| Ru & N |
________________________ |
________________________ |
| Ni & Cu |
________________________ |
________________________ |
| Be & S |
________________________ |
________________________ |
Did the two methods give different results?
Polarity Of Bonds
When calculating the electronegativity difference to determine whether a given
bond is covalent or ionic, it may have occured to you that sometimes the difference
may be barely enough to be ionic or covalent. What if the difference
is exactly 1.67? Well, it helps to remember that even in the most ionic
bond, there is still a little bit of time the "lost" electron spends
around the positive ion. As the difference in electronegativity approaches zero,
the sharing of the electron becomes more and more even, and the bond becomes
less and less ionic.
To clear up these possibilities a little more, we call any bond between two
non-metals with the same electronegativity a pure covalent
bond. If the atoms have similar (but different) electronegativities, they are
said to form a polar covalent bond. In these cases, the electron
spends a bit more time closer to the more electronegative atom. For example,
in water, the H-O bond is polar, with the oxygen "hogging" the electron.
When the hogging is extreme, we have an ionic bond.
So what if the difference is 1.68? Or 1.66? Well, call it what the calculation
tells you (ionic for the first one, covalent for the second), or use the rule
of thumb, and show how you reached your decision. It is unlikely anyone will
argue with your logic.
[http://www.sparknotes.com/chemistry/bonding/intro/][MHS
Chem page]

